Tip: Calibration Procedure for Micropipettes
SCOPE
This procedure describes the method generally used to calibrate all micropipettes. Check the individual manufacturer specifications for acceptable error ranges at nominal volumes.
RECOMMENDED FREQUENCY
Every 3 months
REAGENT REQUIREMENTS
• Distilled Water
PROCEDURE
1.1 Each pipette should be tested at its maximum capacity, and again at no more than 20% of its total capacity (preferably its absolute minimal volume, but this will depend on the size of pipette). If time allows, also do a check at 50% capacity.
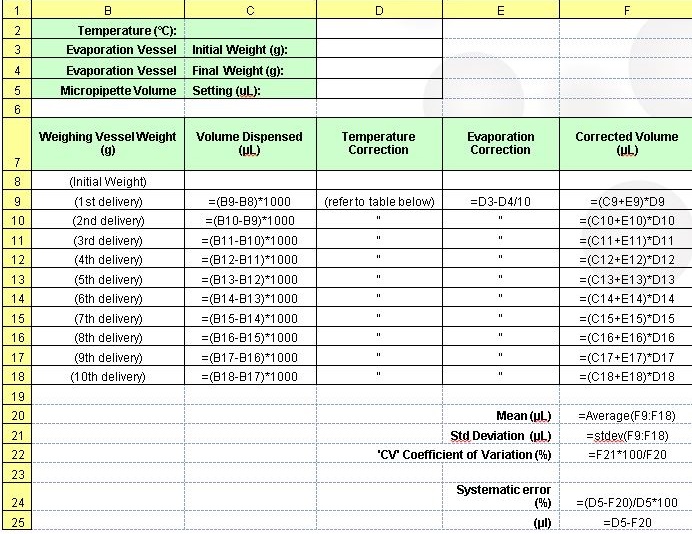
1.2 Create a spreadsheet similar to the one below to record data obtained through this calibration.
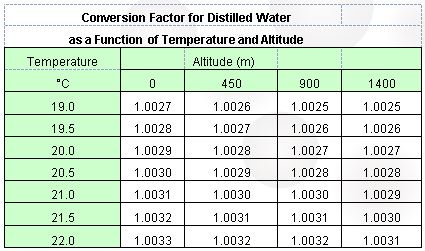
1.3 De-gas approximately 500mL of distilled water, and carefully decant into a 500mL beaker containing a thermometer of known accuracy. Record the temperature of the water – ideal testing temperature is between 19-22˚C. Relative humidity should be between 45-75%, and the approximate site altitude above sea level should be known. (Refer to table below for temperature and altitude correction factor.) Ensure there are no vibrations or air currents around the analytical balance used for weighing.
1.4 Obtain 2 identical weighing vessels, preferably smaller than 50 times the volume to be tested. (For volumes 100μL, the weighing vessels should be capped). Partially fill one with some of the de-gassed distilled water. Weigh this container on a 4-figure balance and record the weight. This will be the evaporation vessel.
1.5 Weigh the second vessel and record this initial weight sheet. This will be the weighing vessel.
1.6 Before proceeding with the calibration, pre-rinse the pipette tip by aspirating and discharging the test water a couple of times.
1.7 Deliver a sample of water into the weighing vessel and record the weight.
1.8 Repeat with another 9 deliveries of water, recording the weight after each delivery.
1.9 Remove the weighing vessel and replace with the evaporation vessel. Record the final weight of the evaporation vessel.
2.0 The spreadsheet will calculate the mean volumes dispensed, standard deviations and CV. Determine if the precision and accuracy meets manufacturer’s specifications.
2.1 If the results are unacceptable, the pipette is to be taken out of service, and maintenance performed until acceptable calibration results can be obtained.
REFERENCE
Australian Standard 2162.2 – 1998. Verification and use of volumetric apparatus – Guide to the use of piston-operated volumetric apparatus (POVA)
Download the Calibration Procedure for Micropipettes Factsheet as a PDF
© Copyright 2014 Vintessential Laboratories. All rights reserved. No part of this publication may be copied or reproduced by any means without the written permission of Vintessential Laboratories
